Original content, please contact the staff and indicate the source in the reproduced article, otherwise infringement will be pursued!
In electrochemical and photocatalytic reactions, an ideal catalyst can exhibit high current density at relatively low overpotentials. Tafel slope provides crucial insights for exploring reaction mechanisms, especially in elucidating the rate-determining steps and reaction pathways.
In electrochemical and photocatalytic experiments, kinetic relationships are generally represented by the Butler-Volmer equation[1]:
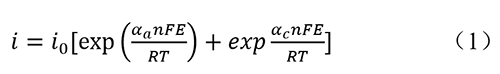
i: Current density
i0: Exchange current density
αa: Anodic electron transfer coefficient
αc: Cathodic electron transfer coefficient
n: Number of electrons transferred in the reaction
F: Faraday's constant
E: Applied voltage
R: Universal gas constant
T: Thermodynamic temperature
At high anodic potentials, the current mainly comes from the anodic current, and the cathodic current can be neglected, simplifying equation (1) to
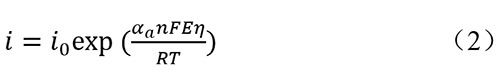
Where η represents overpotential, and equation (2) is also known as the Tafel equation. Tafel slope can be obtained from the LSV curve. The Tafel slope can also be expressed as:
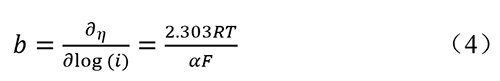
From this, it can be seen that the smaller the Tafel slope, the faster the increase in current density, indicating faster catalytic kinetics and better catalytic activity.
How can we infer the reaction mechanism based on the experimentally measured Tafel slope?
First, use the Tafel slope to infer the rate-controlling step of the reaction. Generally, photocatalytic reaction experiments require testing the performance improvement of the working electrode for processes like HER, OER, or CO₂RR.
During testing, check the open-circuit "potential-time curve." When the test system is still for 15 minutes and the open-circuit potential is stable, you can start testing the Tafel curve. The lowest point of the Tafel curve will be lower than the open-circuit potential. It is recommended to subtract 0.1 V from the open-circuit potential as a reference. A smaller scan speed and longer test time will result in more accurate results.
It should be noted that Tafel curve testing is highly corrosive, and each sample can only be tested once. It is recommended to test the Tafel curve last after other non-corrosive tests have been completed. If the results are not ideal, you need to prepare the sample again and replace the electrolyte for retesting.
![图1. 经典Tafel方法在非氧化还原缓冲体系中应用原理图[2].jpg 图1. 经典Tafel方法在非氧化还原缓冲体系中应用原理图[2].jpg](https://perfectlight.cn/Uploads/UserFile/Image/1/20220715/62d17d111b17b.jpg)
Figure 1. Application schematic of the classic Tafel method in non-oxidation-reduction buffer systems[2]
According to the reaction mechanism, I1,a and I2,a in Figure 1 represent the cathodic slope and anodic slope, respectively, obtained by extrapolation. There are mainly two methods for fitting:
① Manual calculation
Use the Origin software with the Tafel Extrapolation plugin installed for calculation. It is important to note that when fitting the data, use log(i) as the X-axis and E as the Y-axis; otherwise, the slope obtained will be the reciprocal of the actual slope.
② Automatic calculation
Use the software provided by the electrochemical workstation, which is the most convenient method.

Figure 2. Tafel plots[3-4]
Through LSV, Tafel curves can be calculated to further reveal the catalytic kinetic information of HER. For HER, the theoretical Tafel slopes are 120 mV/dec, 40 mV/dec, and 30 mV/dec, corresponding to the Volmer-Heyrovsky mechanism, Heyrovsky mechanism, and Tafel mechanism[5].
For the Volmer-Heyrovsky mechanism in the HER reaction:
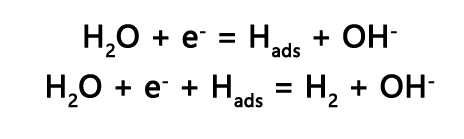
A smaller Tafel slope means faster kinetic processes, indicating that the catalyst can achieve the required current at lower overpotentials.
[1] Stephan Enthaler*, Jan von Langermann*, Thomas Schmidt*. Carbon Dioxide and Formic Acid-the Couple for Environmental-Friendly Hydrogen Storage? [J]. Energy Environmental Science, 2010, 3, 1207.
[2] 秦越强,左勇,申淼. FLiNaK-CrF3/CrF2氧化还原缓冲熔盐体系对316L不锈钢耐蚀性能的影响[J].中国腐蚀与防护学报, 2020, 40(02):182.
[3] Ya Zhang, Lang Hu, Yongcai Zhang*, et.al. NIR Photothermal-Enhanced Electrocatalytic and Photoelectrocatalytic Hydrogen Evolution by Polyaniline/SnS2 Nanocomposites[J]. ACS Applied Nano Materials, 2022, 5: 391.
[4] Priti Sharma, Debdyuti Mukherjee, Yoel Sasson*, et. al. Pd doped carbon nitride (Pd-g-C3N4): an efficient photocatalyst for hydrogenation via an Al-H2O system and an electrocatalyst towards overall water splitting[J]. Green Chemistry, 2022, DOI: 10.1039/d2gc00801g.
[5] Guoqiang Zhao, Kun Rui, Wenping Sun*, et. al. Heterostructures for electrochemical hydrogen evolution reaction: a review [J]. Advanced Functional Materials, 2018, 28(43): 1803291.
The above content is translated and summarized by the author based on reference materials. The author's proficiency is limited, so if there are any errors, please feel free to correct them. Original content is not easy to produce; if you intend to repost this article, please contact the staff and include the source information in the reposted article, or else copyright infringement will be pursued!