Original content is not easy; if you wish to reproduce this article, please contact the staff and include a source reference in the reproduced article; otherwise, infringement will be pursued!
Photocatalysis is the combination of photolysis and electrocatalysis, maximizing the advantages of both technologies.
Compared to photolysis, in photocatalytic reactions, the photoelectrode can utilize sunlight to generate photogenerated electrons, enhancing reaction activity and catalytic efficiency.
Compared to electrocatalysis, photocatalytic reactions significantly reduce the external energy input, effectively reducing energy consumption and environmental pollution.
Photocatalysis can also make catalysts unsuitable for photocatalysis due to band structure mismatch suitable for photocatalysis under appropriate external voltage conditions.
Photocatalytic reactions can be categorized into various types, including photocatalytic water splitting for hydrogen production, photocatalytic degradation, photocatalytic CO₂ reduction, photocatalytic nitrogen reduction for ammonia synthesis, and more, depending on the reactants and reaction types.
Photocatalytic reactions are electrochemical processes under the influence of light and involve the excitation of electrons due to light absorption, resulting in charge transfer. Semiconductor materials have a weak interaction between valence band electrons and conduction band electrons due to their bandgap. Upon excitation by light, valence band electrons of the semiconductor move to the conduction band, leaving behind holes in the valence band, initiating photocatalytic reactions.

Figure 1. Three common types of photocatalytic reaction cells (a) Photoanode photocatalytic reaction cell; (b) Cathode photocatalytic reaction cell; (c) Series-connected photocatalytic reaction cell.

Photocatalysis combines the principles of photolysis and electrocatalysis, maximizing the advantages of both technologies.
The photocatalytic reaction cell based on semiconductor materials mainly consists of four parts:
1. Photocatalytic Electrode: Also known as the working electrode, it is the core component of the photocatalytic reaction cell, composed of semiconductor materials used to capture light energy.
In general, the n-type semiconductor film/conductive substrate is called the photoanode, and the p-type semiconductor film/conductive substrate is called the photocathode.
The common method for preparing photocatalytic electrodes is to attach photocatalytic semiconductor materials to transparent conductive oxide-coated glass with a low work function, such as indium tin oxide (ITO) or fluorine-doped tin oxide (FTO). The photocatalytic electrode is clamped using an electrode fixture as shown in Figure 2(a).
2. Counter Electrode: When studying a single photoanode or photocathode, a counter electrode is introduced to serve as the other half of the half-reaction site. The counter electrode is typically made of Pt electrode, as shown in Figure 2(b).
3. Reference Electrode: To ensure the accurate and stable potential applied to the photocatalytic electrode, a reference electrode is commonly added, creating a three-electrode system for the entire photocatalytic reaction cell. Commonly used reference electrodes include Ag/AgCl electrodes, Hg/Hg₂Cl₂ electrodes, or Hg/HgO electrodes, as shown in Figure 2(c).
4. Appropriate Electrolyte Solution: The electrolyte solution serves to establish an ionic conductivity circuit in the photocatalytic reaction cell. It should possess good ionic conductivity.
Depending on the properties of the photocatalytic electrode material in the photocatalytic reaction, the pH value and the ionic composition of the electrolyte solution vary. When selecting an electrolyte solution, it's essential to ensure that the chosen electrolyte solution does not react with the selected reference electrode. Electrolyte solutions can be divided into three types:
1) Acidic electrolyte solutions: Such as H₂SO₄ solution;
2) Neutral electrolyte solutions: Such as Na₂SO₄ solution, K₂SO₄ solution, etc.;
3) Alkaline electrolyte solutions: Such as NaOH solution, KOH solution, etc.
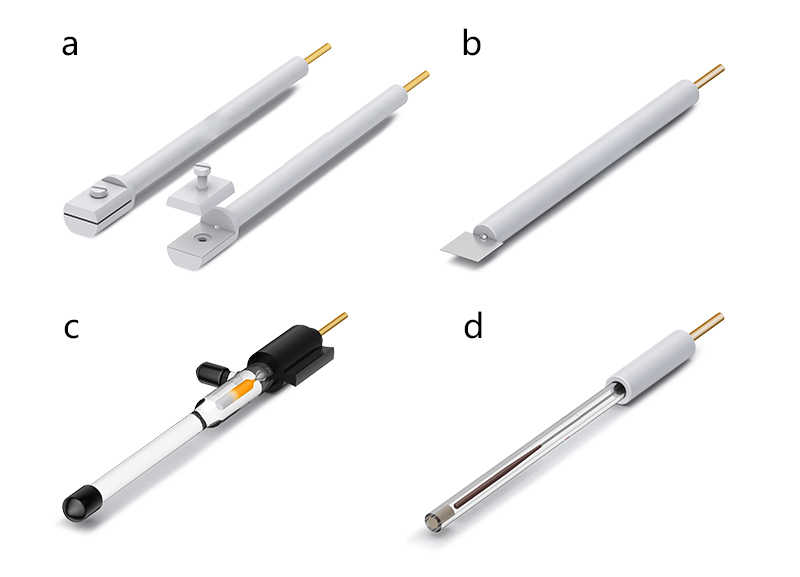
Figure 2. (a) Electrode fixture; (b) Pt electrode; (c) Hg/Hg₂Cl₂ electrode; (d) Ag/AgCl electrode.
The above content is a translation and summary based on reference materials. The author's knowledge is limited, so if there are any errors, please feel free to correct them!