Originality is not easy; if you wish to republish this article, please contact the staff and indicate the source in the republished article, otherwise it will be treated as an infringement!
Photocatalytic CO₂ reduction has characteristics such as being green, mild in conditions, and having abundant raw materials. Therefore, it is considered an effective approach to achieve "carbon peak" and "carbon neutrality." Constrained by conversion rate and selectivity issues, current research on photocatalytic CO₂ reduction is still in the laboratory stage. In addition to developing and designing efficient catalysts, optimizing reaction processes and changing reaction conditions can also achieve efficient conversion of CO₂ through photocatalysis.
In general, photocatalytic CO₂ reduction mainly occurs in the gas phase or liquid phase[1].
The liquid-phase reaction system occurs in a saturated CO₂ solution, where the photocatalyst is evenly dispersed in the solution.
The gas-phase reaction system involves fixing the photocatalyst on a substrate, and a mixture of CO₂ and water vapor directly reacts with the photocatalyst, as shown in Figure 1[2].
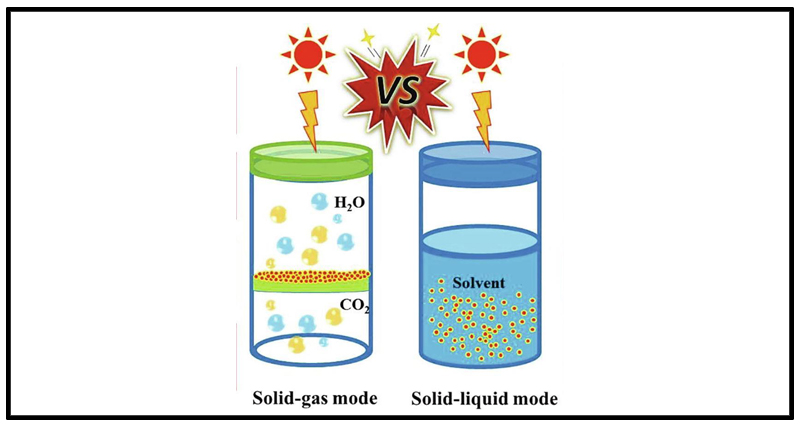
Figure 1. Comparison of gas-phase and liquid-phase photocatalytic CO₂ reduction reaction models[2].
In the liquid-phase reaction system, due to the solid catalyst dispersed in the solution always being in a stirred state, the efficiency of charge transfer and heat transfer is higher[3, 4]. However, in the liquid-phase reaction system, the limited solubility and diffusion coefficient of CO₂ in H₂O can limit the mass transfer efficiency of photocatalytic CO₂ reduction[5].
At 25℃ and 101.325 kPa reaction conditions, the solubility of CO₂ in H₂O is less than 0.033 mol·L-1, which reduces the diffusion of CO₂ molecules from the gas phase to the photocatalyst surface[6].
Compared to neutral and acidic conditions, the solubility of CO₂ in alkaline conditions is higher[1]. This can be achieved by increasing the solution's pH value to enhance CO₂ solubility, or by adding organic solvents such as acetonitrile (ACN)[7] and ethyl acetate (EAA)[8] to H₂O to facilitate CO₂ dissolution.
To address the above issues, researchers have proposed conducting photocatalytic CO₂ reduction in the gas phase. Compared to liquid-phase reactions, gas-phase reactions are not affected by factors such as sacrificial agents, photosensitizers, and solvents, making them a relatively simple reaction system.
The diffusion coefficient of CO₂ in the gas phase is approximately 0.1 cm2·s-1, which is about four orders of magnitude higher than in the liquid phase[9, 10]. Therefore, in gas-phase reactions, the mass transfer efficiency between CO₂ and the photocatalyst is higher.
Another advantage of gas-phase photocatalytic CO₂ reduction is the effective suppression of hydrogen evolution reactions[2, 11]. Since the reduction of H₂O to H₂ is more favorable thermodynamically and kinetically, photocatalytic CO₂ reduction reactions conducted in the liquid phase may induce hydrogen evolution reactions, reducing the CO₂ conversion rate[1, 6]. Gas-phase photocatalytic CO₂ reduction effectively addresses this issue.
Currently, gas-phase photocatalytic CO₂ reduction reactions are mainly divided into two methods. One is to coat the photocatalyst on a substrate, forming a thin film, and CO₂ with a certain humidity flows over the top layer of the thin film, as shown in Figure 2(a). The other method is a fixed-bed gas-phase reaction, where CO₂ with a certain humidity passes directly through the photocatalyst bed, as shown in Figure 2(b). Compared to the first method, fixed-bed systems have more efficient mass transfer, which helps improve the CO₂ conversion rate.
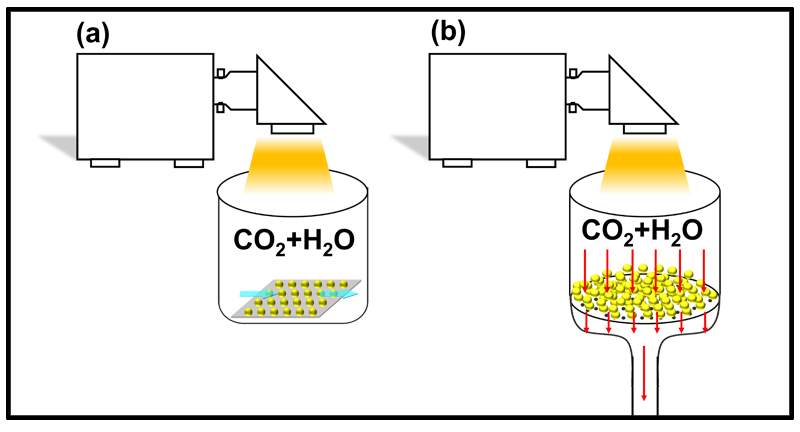
Figure 2. (a) Thin film gas-phase reaction mode and (b) fixed-bed gas-phase reaction mode.
In order to meet the requirements of gas-phase photocatalytic CO₂ reduction, Perfectlight Technology Co., Ltd. has launched an online temperature measurement gas-solid photocatalytic reactor. This reactor is mainly designed for our Labsolar-6A all-glass automatic online trace gas analysis system[12], as shown in Figure 3.
Unlike passive diffusion, the online temperature measurement gas-solid reaction reactor adopts a gas "penetration" scheme, combined with the magnetic drive plunger pump in the Labsolar-6A system, allowing CO₂ to fully contact the catalyst, improve mass transfer efficiency, and enhance reaction conversion.
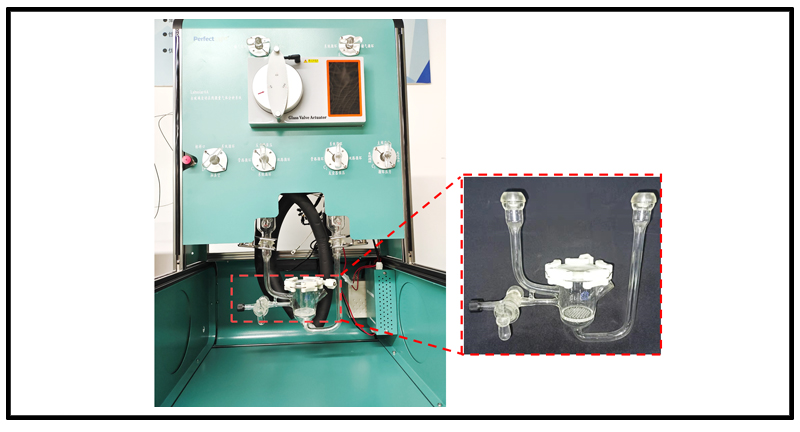
Figure 3. Field photo of the online temperature measurement gas-solid photocatalytic reactor mounted on the Labsolar-6A system (Hunan University)[12].
Aside from photocatalytic CO₂ reduction, the online temperature measurement gas-solid photocatalytic reactor is also suitable for photo-thermal CO₂ reduction reactions. The reactor is equipped with a dedicated in-situ infrared temperature measurement port, which non-contactingly measures the surface temperature of the catalyst in real-time and records it. Additionally, it comes with a constant temperature jacket to minimize heat dissipation, as shown in Figure 4.

Figure 4. Field photo of the Labsolar-6A system with the online gas-solid photocatalytic reactor.
Basic Parameters of the Online Temperature Measurement Gas-Solid Photocatalytic Reactor:
Reactor Material: The reactor is made of high borosilicate glass, and the optical window is made of quartz glass;
Powder Catalyst Placement: Spread on the surface of the reactor's own quartz filter membrane;
Reactor Volume: Total volume (with cantilever): 118 mL; cylindrical part volume: 96 mL;
Reactor Dimensions: Flange outer diameter 60 mm, total height approximately 200 mm;
Temperature Measurement Range: 0~600℃;
Measurement Accuracy: 0.1℃.

Figure 5. Online temperature measurement gas-solid photocatalytic reactor and its accessories.
The above content is a translation and summary based on reference literature by the author. The author's level is limited, so if there are any errors, please correct them!