Original work is not easy. If you want to reprint this article, please contact the staff and include the source information in the reprinted article, otherwise it will be treated as infringement!
Photophysical reactions are widespread in nature and daily life. Typical examples of photophysical reactions include photosynthesis in plants, human visual imaging, synthesis of plastics, fading of dyes under light, and photolytic smoke. Therefore, humans have been aware of photophysical reactions for a long time.
The process of photophysical reactions involves the absorption of light, excitation, and chemical reactions, making it an interdisciplinary study between chemistry and physics. Due to limitations in light sources and separation analysis techniques, it was not until the 1960s that organic photochemistry began to develop.
Compared to traditional thermal chemical reactions, photophysical reactions differ significantly in terms of reaction conditions, reaction pathways, and reaction products.
Differences between Photophysical and Thermal Chemistry
The principle of thermal chemical reactions is illustrated in Figure 1. The collision energy distribution of reactant molecules X follows the Boltzmann distribution, and molecules with collision energy greater than the activation energy Ea have a chance to produce reaction products Y through collision. When X is heated, more molecules move to the high-energy region, increasing the number of compound molecules with collision energy greater thanEa, and the reaction proceeds faster with higher temperature.
The role of a catalyst is to reduce the activation energy required for the reaction, allowing more reactant molecules X to produce Y through collisions at the same temperature. It is a ground-state reaction, like going from one side of a mountain range to the other, and the endpoint is determined by the mountain crossed. The mountain to be crossed is determined by its height, and the optimization of the reaction process is mainly to reduce the height of the target peak (Ea), allowing more compound molecules to climb over the target peak to produce the target product. Therefore, thermal chemical reactions are greatly influenced by temperature, and the reaction pathway and reaction product are relatively uniform.
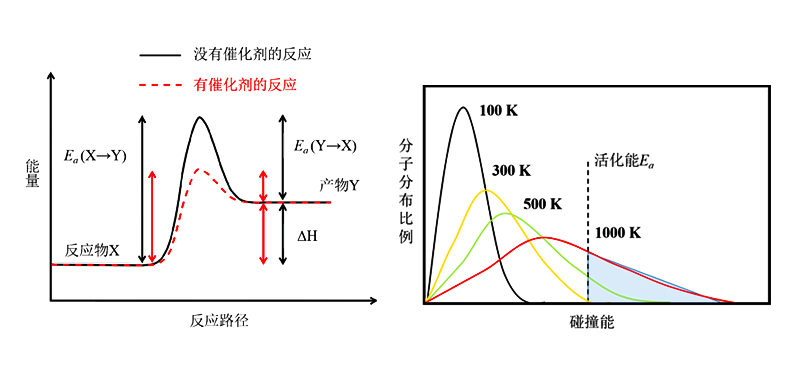
Figure 1 Principle of Thermal Chemical Reactions (Left) and Collision Energy Distribution of Compound Molecules (Right)
Photophysical reactions are excited-state reactions. Reactant molecules absorb light energy and are excited to an excited state, which can be likened to throwing a compound directly to the peak of a mountain. Excited-state molecules can be located at the peaks of any mountains in the range, so the reaction path (mountains crossed) and the selection of reaction products (endpoints) are more numerous and complex than thermal chemical reactions. The movement of reactant molecules from the starting point to the peak of the mountain is mainly related to light absorption, so it is not significantly affected by temperature but by the wavelength and intensity of light.
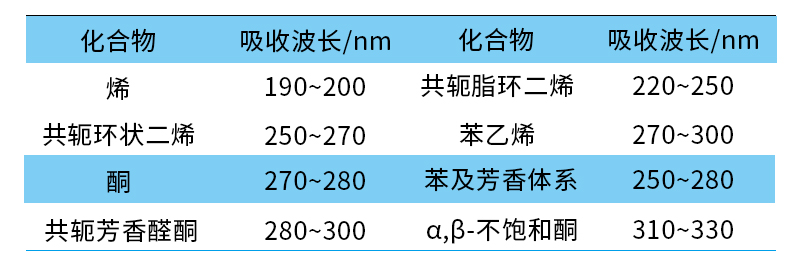
The wavelength range involved in photophysical reactions is between 100 and 1000 nm (with 200 to 700 nm as the main range), and the absorption wavelengths of common organic compounds are shown in Table 1.
The energy of light ε can be calculated using formula (1). Taking light at 300 nm as an example, its energy is approximately 433 kJ/mol, which is much higher than the dissociation energy of σ C-C bonds at 347 kJ/mol. This indicates that excited-state molecules after light absorption have very high internal energy and can initiate many types of organic chemical reactions.

Perfectlight Technology's PCX-50C Discover Multi-channel Photocatalytic Reaction System and PLR-SMCR1000 Multiphase Microchannel Reaction System are equipped with LED light sources, which can meet the experimental requirements for light source wavelength and intensity in different organic photochemical synthesis reactions.

The optical power of LED light source can be adjusted in the range of 100 to 450 mW/cm2, which is ideal for photochemical synthesis experiments with slow reactions at low light power.
Available wavelengths for LED light source:
① Ultraviolet region - can be used for substrate-initiated radical chain reactions
λ=255, 275 nm
② Visible region - achieves visible light-induced catalytic initiation of free radical reactions while reducing side reactions initiated by substrate excitation, improving reaction selectivity
λ=365, 385, 405, 410, 420, 435, 445, 450, 460, 475, 485, 505, 520, 525, 535, 550, 575, 590, 595, 620, 625, 630, 655, 685, 700, 730, 760, 770 nm
③ Visible region - simulates reactions under sunlight conditions
780≥λ≥380 nm
For the most common blue and green light absorption regions of photocatalysts, there are absorption wavelengths such as 450, 460, 475, 485, 505, 520, 525, 535, 550, and 575 nm available for selection. These wavelengths can be combined and precisely controlled to obtain the optimal light wavelength.
Each light source is equipped with optical lenses and individually selected and locked onto the focal plane to ensure consistency and utilization of light source output.
Calculation of Quantum Yield in Photochemistry
Quantum yield φ is typically used to evaluate the efficiency of light energy utilization in photochemical reactions, defined as the ratio of the number of molecules undergoing the reaction to the number of absorbed photons. Since the actual number of photons absorbed by reactants cannot be determined, we usually assume a 100% absorption rate and use the measured number of emitted photons from the light source as the actual number of absorbed photons. The specific calculation formula (2) is as follows:
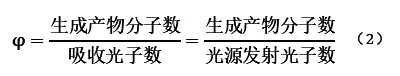
Usually, φ < 1. However, for chain reactions initiated by photosensitizers, one photon can initiate a series of chain reactions, and the energy absorbed by one photon can be transferred among multiple reactant molecules. In such reactions, φ > 1. Therefore, chain reactions initiated by photosensitizers have significant practical industrial applications.
Compared to other types of organic reactions, the study of organic photochemical reactions started relatively late, and due to the difficulty in controlling the reaction pathways and the variability of reaction products of excited-state molecules, these reactions are currently less applied in industrial settings. With a deeper understanding of organic photochemical reactions, photocatalytic oxidation-reduction technology using visible light for single-electron transfer reactions has flourished[1-4]. Through rational design, active organic free radical intermediates can be selectively generated, leading to selective oxidation-reduction reactions that produce target products difficult to synthesize using traditional thermal catalysis.
Currently, the relatively mature types of organic photochemical reactions include the following:
(1) Isomerization, addition, and rearrangement of olefins;
(2) Addition, substitution, and side-chain rearrangement of aromatic compounds;
(3) Dehydrogenation and reduction reactions of carbonyl compounds, photolysis reactions, and addition reactions with olefinic compounds, among others.
These reactions are all chemical reactions initiated directly by organic reaction substrates absorbing light, mainly utilizing ultraviolet light.
Table 1 Common Absorption Wavelengths of Organic Compounds
[1] M. H. Shaw, J. Twilton and D. W. C. Macmillan*, Photoredox Catalysis in Organic Chemistry[J], Journal of Organic Chemistry, 2016, 81(16), 6898.
[2] D. M. Arias-Rotondo and J. K. Mccusker*, The Photophysics of Photoredox Catalysis: A Roadmap for Catalyst Design[J], Chemical Society Reviews, 2016, 45(21), 5803.
[3] S. Reischauer and B. Pieber*, Emerging Concepts in Photocatalytic Organic Synthesis[J], Iscience, 2021, 24(3).
[4] J. Twilton, C. Le, P. Zhang, M. H. Shaw and D. W. C. Macmillan*, The Merger of Transition Metal and Photocatalysis[J], Nature Reviews Chemistry, 2017, 1(7).