As of June 2023, the atmospheric CO₂ concentration has risen to 424 ppm, making the conversion and utilization of CO₂ an urgent matter[1]. In recent years, research on electrocatalytic CO₂ reduction has made significant progress, enabling the conversion of CO₂ into CO, HCOOH, CH₄, C₂H₅OH, and more through electrocatalysis[2~6]. However, the current state of research in electrocatalytic CO₂ reduction still falls short of meeting the requirements for industrial-scale production, with numerous challenges awaiting resolution[7]:
1. Product selectivity remains relatively low, especially for high-value chemicals. Lower selectivity increases the difficulty of product separation and purification;
2. The local current density of the target products is low, and a high overpotential is required. Low current density and high overpotential directly impact the energy conversion efficiency of the electrocatalytic CO₂ reduction reaction;
3. Further research is needed on the reaction mechanism of electrocatalytic CO₂ reduction;
4. The reaction apparatus still cannot meet industrial demands, including electrode durability, ion exchange membrane performance, catalyst stability, and more.

The current reaction cells for electrocatalytic CO₂ reduction mainly include H-type cells, Flow Cells, and Membrane Electrode Assembly (MEA)[8].

Figure a. Schematic of H-type Cells[8].
H-type cells consist of cathode chambers, anode chambers, and ion exchange membranes. CO₂ is continuously introduced into the cathode chamber, where the reduction reaction occurs. The gaseous products, along with CO₂, enter the gas chromatograph for detection, while the anode chamber primarily undergoes oxygen evolution reactions. Typically, 0.5 M KHCO₃ is used as the electrolyte in H-type cells. These cells have a relatively simple design and low cost. However, due to the limited solubility and diffusion coefficient of CO₂ in the electrolyte, mass transfer efficiency is low, resulting in slow reaction rates. The current density of electrocatalytic CO₂ reduction in H-type cells is generally below 100 mA/cm2 [9]. Additionally, neutral electrolytes cannot effectively suppress the HER reaction.
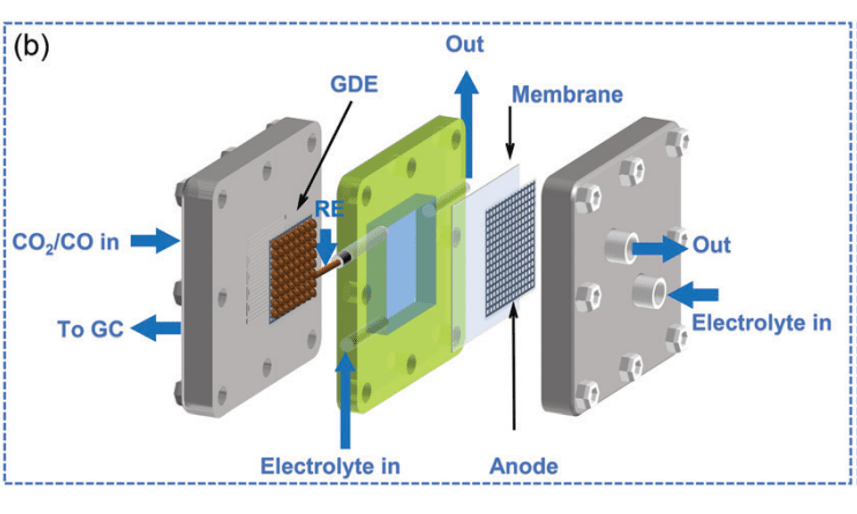
Figure b. Schematic of Flow Cells[8].
Flow cells mainly consist of porous hydrophobic gas diffusion layers, cathode chambers, anode chambers, and ion exchange membranes. CO₂ continuously passes through the porous hydrophobic gas diffusion layer, reacting with the catalyst and electrolyte at the gas-liquid-solid three-phase interface. This design effectively addresses the mass transfer limitations seen in H-type cells[10]. Moreover, it reduces the distance between the cathode and anode chambers, lowering the impedance of the electrolyte and the entire reaction system's ohmic drop[11]. Typically, 1 M KOH is used as the electrolyte in flow cells, which effectively suppresses the competing HER reaction. Electrochemical CO₂ reduction in flow cells typically achieves current densities greater than 500 mA/cm2 [11]. However, despite the high current density achieved in flow cells, stability remains an issue. Due to stability concerns with the gas diffusion layer, flow cells are susceptible to electrolyte overflow. Furthermore, direct contact between CO₂ and alkaline electrolyte can lead to carbonate or bicarbonate formation, clogging the gas diffusion layer's gas transport channels and affecting reaction rates[9,11].
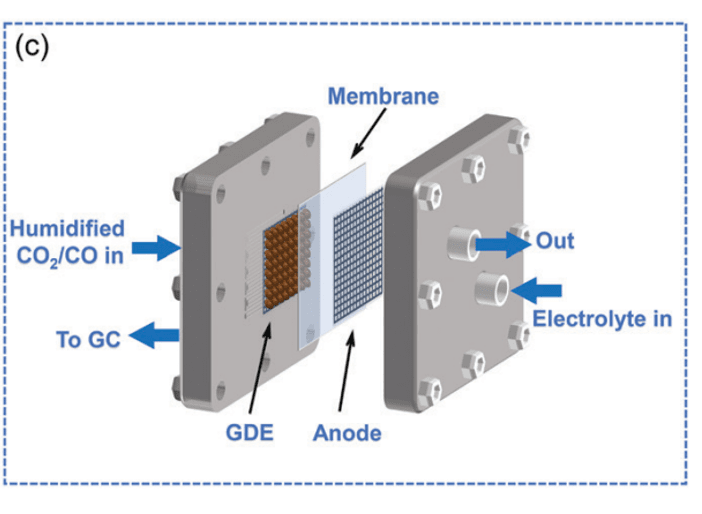
Figure c. Schematic of Membrane Electrode Assembly (MEA)[8].
Membrane electrode assembly (MEA) primarily consists of porous hydrophobic gas diffusion layers, cathode chambers, anode chambers, and ion exchange membranes. MEA retains the excellent mass transfer efficiency seen in flow cells, allowing for high current density. However, unlike flow cells, MEA does not have electrolyte circulating through the cathode chamber. CO₂ gas with a certain humidity directly interacts with the catalyst through porous hydrophobic gas diffusion layers, avoiding issues like electrolyte overflow and gas diffusion layer clogging that occur in flow cells[9,10]. Typically, a certain concentration of KOH is used as the electrolyte in the anode chamber. Since there is no electrolyte in the cathode chamber, no potential circuit forms between the reference electrode and the working electrode in MEA. This differs from H-type cells and flow cells, making it a two-electrode system rather than a three-electrode system. This distinction, combined with the shorter distance between the two chambers, significantly reduces system impedance, improving the reaction rate and energy conversion efficiency of electrocatalytic CO₂ reduction[9,10]. However, MEA still falls short of meeting the requirements for practical industrial-scale production. Due to the absence of electrolyte circulation in the cathode chamber, liquid products from the reduction reaction can easily block the gas transport channels of the porous hydrophobic gas diffusion layer, affecting mass transfer throughout the reaction. Additionally, the limited lifespan of ion exchange membranes does not meet the long-term production requirements[9].
In addition to developing new, efficient, and stable catalysts, the design and development of new reactors are equally important for electrocatalytic CO₂ reduction research.
To meet experimental needs, Perfectlight Technology has introduced the PLS-MECF series dual-chamber alkaline electrolyzer, known for its efficiency, stability, and long lifespan. There are two models available: PLS-MECF-01 dual-chamber alkaline electrolyzer and PLS-MECF-02 visualized dual-chamber alkaline electrolyzer. For more information, please call 400-1161-365 for inquiries or contact our online customer service.
The content above is a translation and summary based on reference literature. I have limited expertise, so please feel free to correct any errors!
[1] Charles D Keeling, Alane F. Carter, Willem G. Mook, Scripps institution of oceanography[J]. 2023.
[2] Yu Ke, Sun Kainan, Chen Chen* et.al., Oxalate-Assisted synthesis of hollow carbon nanocage with Fe single atoms for electrochemical CO2 reduction [J]. Small, 10.1002/smll.202302611.
[3] Zang Yipeng, Liu Tianfu, Wang Guoxiong* et.al., In situ reconstruction of defect-rich SnO2 through an analogous disproportionation process for CO2 electroreduction[J]. Chemical Engineering Journal, 2022, 5: 446.
[4] Deng Bangwei, Huang Ming, Dong Fan* et.al., The crystal plane is not the key factor for CO2-to-methane electrosynthesis on reconstructed Cu2O microparticles[J]. Angewandte Chemie International Edition, 2021, 61: 7.
[5] Wang Pengtang, Huang Xiaoqing*, Qiao Shizhang* et.al., Boosting electrocatalytic CO2-to-ethanol production via asymmetric C-C coupling[J]. Nature Communications, 2022, 13: 3754.
[6] Mangjeet Chhetri, Che Fanglin*, Yang Ming* et.al., Dual-site catalysts featuring platinum-group-metal atoms on copper shapes boost hydrocarbon formations in electrocatalytic CO2 reduction[J]. Nature Communications, 2023, 14: 3075.
[7] She Xiaojie, Shik Chi Edman Tsang*, Shu Ping Lau* et.al., Challenges and opportunities of electrocatalytic CO2 reduction to chemicals and fuels[J]. Angewandte Chemie International Edition 2022, 22: 49.
[8] Ma Wenchao, Xie Shunji*, Wang Ye* et.al., Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts[J]. Chemical Society Reviews, 2021, 50: 12897.
[9] Yuan Lei, Zeng Shaojuan, Zhang Suojiang* et.al., Advances and challenges of electrolyzers for large-scale CO2 electroreduction[J]. Materials Reports: Energy, 2023, 3. 100177.
[10] Xu Dezhi, Liu Xue*, Ma Tianyi* et.al., Electrocatalytic CO2 reduction towards industrial applications[J]. Carbon Energy, 2023, 15: 230.
[11] Lai Wenchuan, Lin Zhiqun*, Huang Hongwen* et.al., Design strategies for markedly enhancing energy efficiency in the electrocatalytic CO2 reduction reaction[j]. Energy Environ. Sci., 2022, 15: 3603.