When the Solar-to-Hydrogen (STH) ratio reaches 5-10%, the photocatalytic water splitting for hydrogen production becomes economically viable[1]. Currently, in research on photocatalytic water splitting for hydrogen production, the most significant activity evaluation parameters include the normalized photocatalytic hydrogen production rate (μmol·h-1·g-1 or mmol·h-1·g-1), quantum yield (AQY), and STH energy conversion efficiency. However, the normalized photocatalytic hydrogen production rate is influenced by factors such as incident light wavelength range, light intensity, reactor type, and reaction temperature, which makes it difficult to compare data results across different laboratories under unified standards[2]. Therefore, the two primary indicators for evaluating catalysts in photocatalytic water splitting for hydrogen production are AQY and STH energy conversion efficiency. Details regarding AQY are elaborated in the article "A Comprehensive Guide to Quantum Yield (AQY) Calculation in Hydrogen Production from Photocatalysis".
STH energy conversion efficiency is the efficiency of converting solar energy into hydrogen energy and is a practical application standard for measuring the ability of photocatalysts to split water[3].
1. The formula for calculating STH energy conversion efficiency in photocatalytic water splitting reactions is as follows[2]:
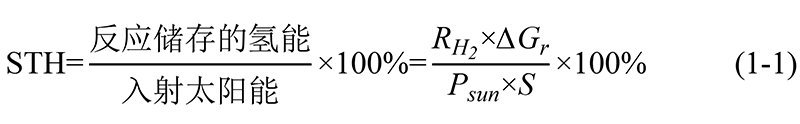
RH₂: Photocatalytic hydrogen production rate (mmol·s-1);
∆Gr: Molar Gibbs free energy of water splitting reaction (J·mol-1);
Psun: Light power density of AM 1.5G standard solar spectrum (100 mW·cm-2);
S: Illuminated area (cm2).
The standard molar Gibbs free energy of water splitting reaction 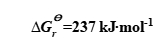 ; Formula (1-1) can be simplified[4]:
; Formula (1-1) can be simplified[4]:
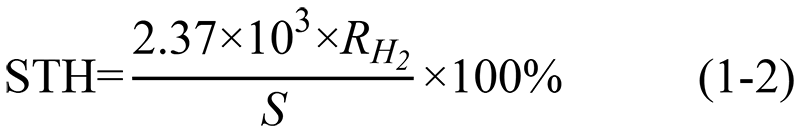
Theoretically, STH energy conversion efficiency can also be calculated by integrating the quantum efficiency over all wavelengths, with the formula as follows[5]:
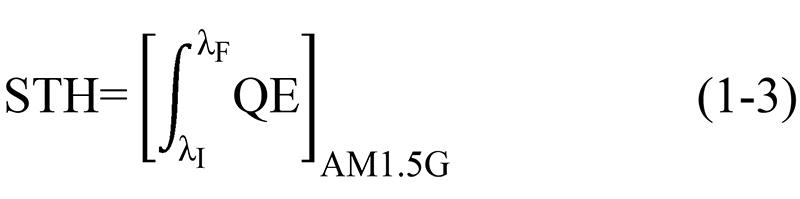
λI: Starting wavelength of AM1.5G standard solar spectrum;
λF: Ending wavelength of AM1.5G standard solar spectrum;
QE: Quantum efficiency under standard conditions.
2. The formula for calculating STH energy conversion efficiency in photoelectrocatalytic water splitting reactions is as follows[6]:
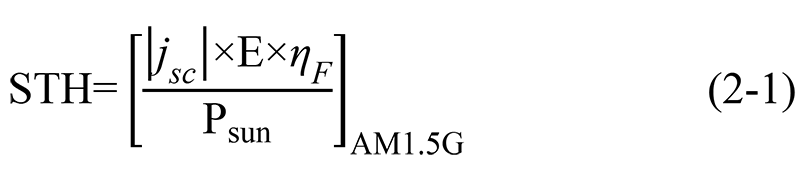
Jsc: Short-circuit photocurrent density (mA·cm-2);
E: Thermodynamic potential for water splitting (V);
ηF: Faradaic Efficiency;
Psun: Photogenerated power density of AM1.5G standard solar spectrum (100 mW·cm-2).
Similarly, the standard thermodynamic decomposition potential of water 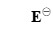 = 1.23 V, and formula (2-1) can be simplified as [6]:
= 1.23 V, and formula (2-1) can be simplified as [6]:
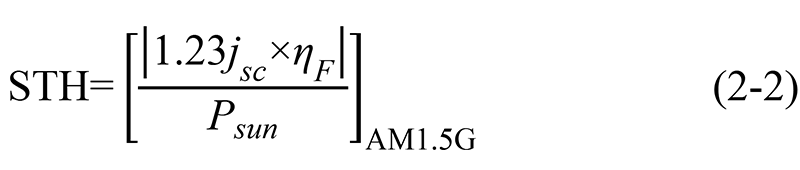
When using the above formulas, the following points should be noted[2]:
1. The calculation of STH energy conversion efficiency is only applicable to the full water splitting reaction with an H₂:O₂ molar ratio of 2:1; it does not apply to the hydrogen evolution half-reaction in the presence of hole sacrificial agents. Since hole sacrificial agents also participate in the reaction, ∆Gr and E values will change;
2. ∆Gr and E values vary at different temperatures and pressures, and they need to be corrected according to the actual reaction temperature and pressure;
3. The light source spectrum must conform to the AM1.5G standard solar spectrum and have a light power density of 100 mW·cm-2.
From the above formulas, it can be concluded that the two necessary conditions for accurately measuring the STH energy conversion efficiency of photocatalysis/photoelectrocatalysis are:
① Accurate measurement of hydrogen production rate in photocatalytic water splitting;
② AM1.5G standard solar spectrum.
Regarding the accurate measurement of hydrogen production rate in photocatalytic water splitting, the reaction system should consider the following interfering factors:
1. Avoid measurement errors caused by offline manual injection;
2. Avoid uneven mixing of H₂ and O₂ in a short time.
Regarding obtaining the AM1.5G standard solar spectrum, there are two common methods: ① using a solar simulator directly; ② using a xenon lamp light source combined with an AM1.5G filter, as shown in Figure 1.
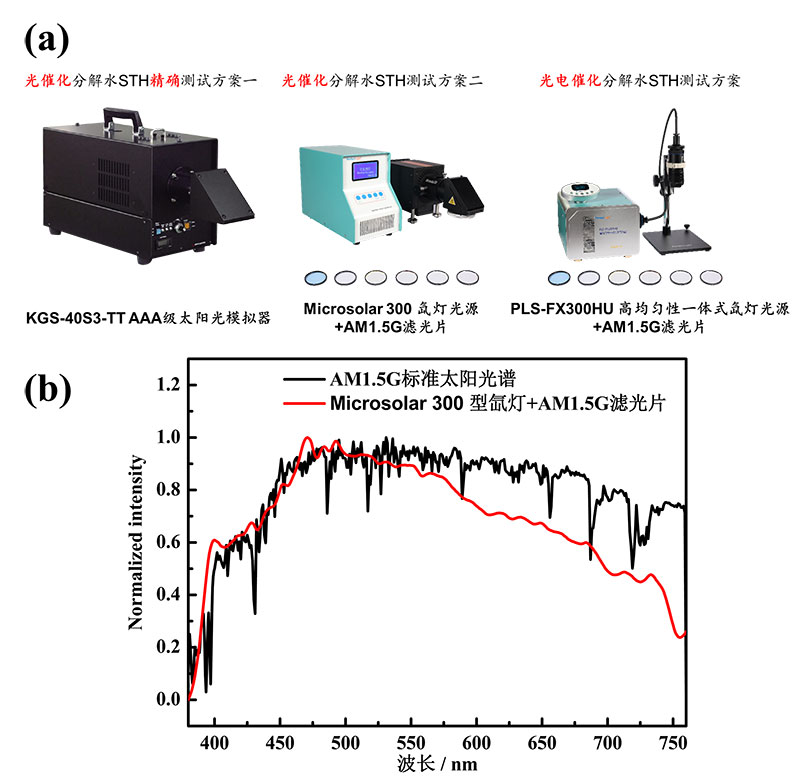
Figure 1. (a) Real photo of solar simulator and xenon lamp combined with AM1.5G filter, (b) AM1.5G standard solar spectrum and Microsolar 300 xenon lamp combined with AM1.5G filter spectrum
Prof. Yongfa Zhu's team from Tsinghua University and Dr. Tierui Zhang's team from the Institute of Chemistry, Chinese Academy of Sciences, used the PLS-FX300HU High Uniformity Xenon Lamp from Beijing Perfectlight as the light source for photocatalytic full water splitting experiments. They used the Beijing Perfectlight Labsolar-6A system to analyze the H₂ and O₂ content online and measure the STH energy conversion efficiency of photocatalytic water splitting. Relevant achievements have been published in Nano Energy[7].

Prof. Jiatao Zhang's team from Beijing Institute of Technology used this scheme and the PLS-FX300HU High Uniformity Xenon Lamp as the light source for photoelectrocatalytic water splitting experiments. They used the Labsolar-6A All-Glass Automatic Online Trace Gas Analysis System to analyze the H₂ content online and measure the STH energy conversion efficiency of photoelectrocatalytic water splitting. Relevant achievements have been published in Advanced Energy Materials[8].

Figure 3. Classic Case - Measurement of STH in Photoelectrocatalytic Water Splitting
[1]Matthew R. Shaner, Nathan S. Lewis*, Eric W. McFarland*, et. al., A comparative technoeconomic analysis of renewable hydrogen production using solar energy[J]. Energy Environmental Science, 2016, 9, 2354.
[2]Wang Zheng, Li Can, Kazunari Domen*, Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting[J]. Chemical. Society. Reviews, 2019, 48, 2109.
[3]Li Rengui, Li Can*, Photocatalytic water splitting on semiconductor-based photocatalysts[J]. Advances in Catalysis, 2017, 60, 1.
[4]Li Yiyang, Wang Zihan, Tsang Shik Chi Edman* et. al., Local magnetic spin mismatch promoting photocatalytic overall water splitting with exceptional solar-to-hydrogen efficiency[J]. Energy Environmental Science, 2022. DOI: 10.1039/d1ee02222a
[5]Qureshi Muhammad, Takanabe Kazuhiro *, Insights on measuring and reporting heterogeneous photocatalysis: efficiency definitions and setup examples[J]. Chemistry of Materials, 2017, 29, 158.
[6]Chen Zhebo, Deutsch Todd G., Jaramillo Thomas F.* et. al., Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols[J]. Journal of Materials Research, 2010, 25, 3.
[7]Chen Xianjie, Zhu Yongfa*, Zhang Tierui* et. al., Three-dimensional porous g-C3N4 for highly efficient photocatalytic overall water splitting [J]. Nano Energy, 2019, 59, 644.
[8]Wang Hongzhi, Guo Yuying, Zhang Jiatao* et. al., Efficient plasmonic Au/CdSe nanodumbbell for photoelectrochemical hydrogen generation beyond visible region[J]. Advanced Energy Materials, 2019, 9, 1803889.