Original content is not easy to create. If you wish to repost this article, please contact the staff and provide proper credit to the source in the reposted article. Otherwise, copyright infringement actions will be taken!
In the field of photocatalytic research, it is common practice to use normalized reaction rates (μmol·h-1·g-1 or mmol·h-1·g-1) to measure the catalytic activity of catalysts. However, due to variations in conditions such as light sources, reactors, and catalyst dosages among different laboratories, normalized reaction rates cannot accurately reflect the photocatalytic activity of the catalyst[1,2]. In different photocatalytic reaction systems, significant differences exist in the catalyst's light absorption coefficient, scattering/reflection effects, and powder suspension level. As a result, the change in reaction rates with increasing catalyst dosage varies among different systems. For the same photocatalytic reaction system, excessive addition of catalyst can affect the penetration depth of incident light and the degree of scattering of incident light by the catalyst, leading to unchanged or even decreased catalytic activity with increasing catalyst dosage, as shown in Figure 1[1]. Based on these reasons, there is a need to find a new evaluation parameter to assess the catalytic activity of photocatalysts under a consistent standard.
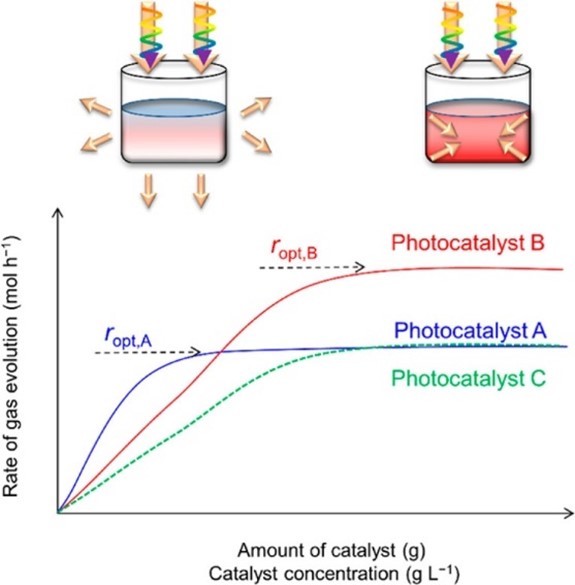
Figure 1. Relationship between gas evolution rate and catalyst dosage[1]
The quantum yield of photocatalysis (Quantum Yield, QY) refers to the ratio of the number of electrons participating in the reaction to the total number of absorbed photons in a system under specific wavelength conditions. However, for non-homogeneous photocatalytic systems, the actual number of photons absorbed by the catalyst is difficult to determine due to factors such as scattering and reflection, making it impossible to directly calculate the quantum yield.
Therefore, the quantum yield of photocatalysis can be calculated by replacing the absorbed photon count with the incident photon count; the quantum yield calculated in this manner is referred to as the "apparent quantum yield" (Apparent Quantum Yield, AQY)[3]. The formula for calculating the apparent quantum yield is as follows[4]:

Ne: Total number of transferred electrons ;
Np: Number of incident photons;
V: Reaction rate (mol·s-1) ;
NA: Avogadro's constant (6.02×1023 mol-1)
K: Number of transferred electrons
h: Planck's constant (6.62×10-34 J·s)
c: Speed of light (3.0×108 m·s-1)
I: Light power density (W·m-2)
A: Incident light area (m2) :
λin: Incident light wavelength (nm)
After simplifying the formula:

For photocatalytic water splitting to produce hydrogen, the number of transferred electrons K = 2[4]
For photocatalytic water splitting to produce oxygen, the number of transferred electrons K = 4
For photocatalytic CO₂ reduction, for products like CO, the number of transferred electrons K = 2[5]
For products like CH₄ in photocatalytic CO₂ reduction, the number of transferred electrons K = 8
For photocatalytic synthesis of ammonia, the number of transferred electrons K = 3[6]
Key factors affecting the accuracy of photocatalytic quantum yield measurement include monochromaticity and photon scattering. From Formula (1), it can be seen that the monochromaticity of incident light directly affects the measurement accuracy of photocatalytic quantum yield. Monochromatic light should have a sufficiently narrow Full Width at Half Maxima (FWHM) to avoid interference from light in other wavelength ranges with the reaction system.
Currently, the most common way to obtain monochromatic light is by using a xenon lamp combined with bandpass filters. However, the FWHM of typical bandpass filters is around 20 nm, leading to significant interference from light in other wavelength ranges.
Laser light sources have a FWHM of less than 5 nm, offering excellent monochromaticity, as shown in Figure 2. Laser-generated monochromatic light is intense and exhibits a linear relationship between intensity and applied current, facilitating precise adjustment of light intensity. Thus, lasers are the most suitable light source for photocatalytic quantum yield measurement.
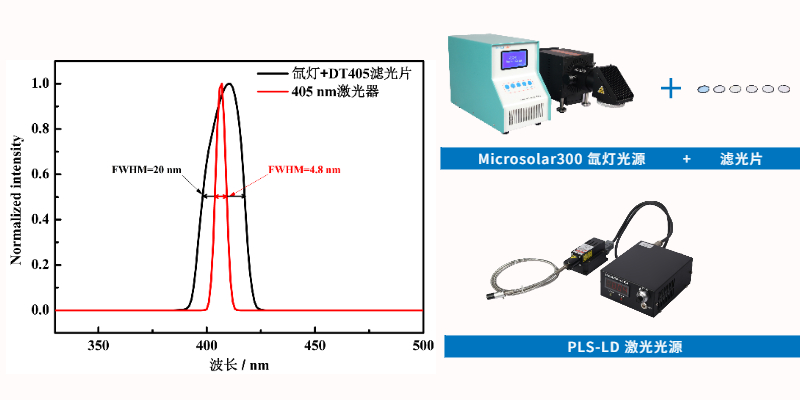
Figure 2. Spectra of xenon lamp combined with 405 nm bandpass filter and 405 nm laser
Measurement of the apparent quantum yield of photocatalysis is carried out under the premise that all incident photons illuminate the reaction system. However, due to photon scattering, the number of photons participating in the actual reaction is reduced, affecting the measurement accuracy of the apparent quantum yield of photocatalysis. Therefore, the reactor used for photocatalytic quantum yield measurement should minimize photon scattering.
Most researchers use glass reactors as reaction units for photocatalytic quantum yield measurement, but the drawback is that incident light can penetrate the glass reactor, causing some photons to not enter the reaction system. While wrapping the reactor with tin foil can mitigate photon scattering, energy loss still occurs due to the rough surface of the tin foil, preventing all photons from entering the reaction system, as shown in the left image of Figure 3.
The ideal reactor for photocatalytic quantum yield measurement is a "blackbody" reactor. This type of reactor employs a spherical silver-coated mirror surface that utilizes total internal reflection principles to completely contain photons within the reactor, effectively preventing photon scattering and enhancing measurement accuracy of photocatalytic quantum yield, as shown in the right image of Figure 3.
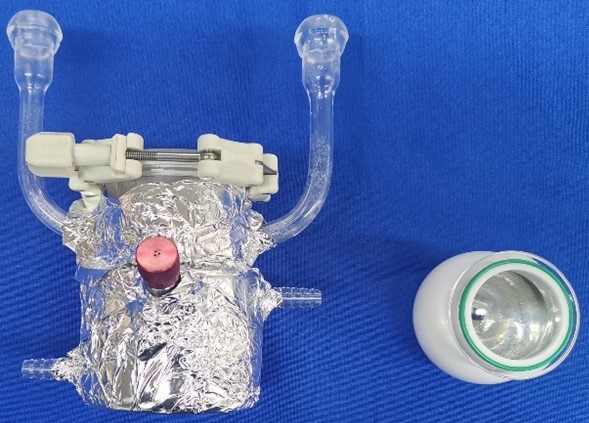
Figure 3. Reactors for photocatalytic quantum yield measurement, reactor wrapped in tin foil (left) and blackbody reactor (right)
Combining "monochromaticity of the light source" and "reactor preventing photon scattering," Beijing Perfectlight Technology Co., Ltd. has introduced the PLR-QY1000 photocatalytic quantum yield measurement system, designed for accurately measuring the apparent quantum yield of photocatalysis. This system utilizes a laser as the light source, ensuring excellent monochromaticity. It incorporates a spherical blackbody reactor that effectively contains all photons within the reaction system. The system features a built-in data processing module, allowing direct measurement of quantum yield, gas production rate, total gas production, and photon count for photocatalysis, as shown in Figure 4.

Figure 4. PLR-QY1000 photocatalytic quantum yield measurement system (left) and system interface (right)
The system is equipped with temperature and pressure control modules, facilitating research on photocatalytic quantum yield under different temperature and pressure conditions.
High-precision sensors are used for rapid determination of H₂ molecules, ensuring measurement accuracy while monitoring the change in catalyst quantum yield over time.
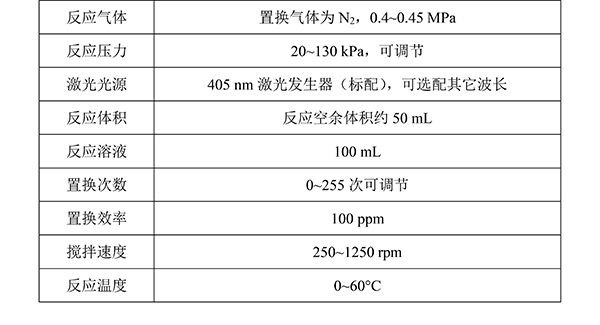
Table 1. Technical specifications of the PLR-QY1000 photocatalytic quantum yield measurement system
Currently, this system has been adopted by major universities and research laboratories both domestically and internationally, with related results published in renowned international journals.
Professor Ma Baojun's research group at Ningxia University, using the PLR-QY1000 photocatalytic quantum yield measurement system equipped with a 532 nm laser, studied the photocatalytic hydrogen production quantum yield of (Pd/MoP)/CdS catalysts in the presence of lactic acid as a sacrificial agent. The results were published in Applied Surface Science[7].
Professor Song Shaoqing and Professor Jiang Shujuan's research group at Ningbo University, using the PLR-QY1000 photocatalytic quantum yield measurement system equipped with 420 nm, 450 nm, and 480 nm lasers, studied the photocatalytic hydrogen production quantum yield of Au/g-C₃N₄ catalysts in the presence of Na₂S/Na₂SO₃ as a sacrificial agent. The results were published in Applied Catalysis B: Environmental[8].
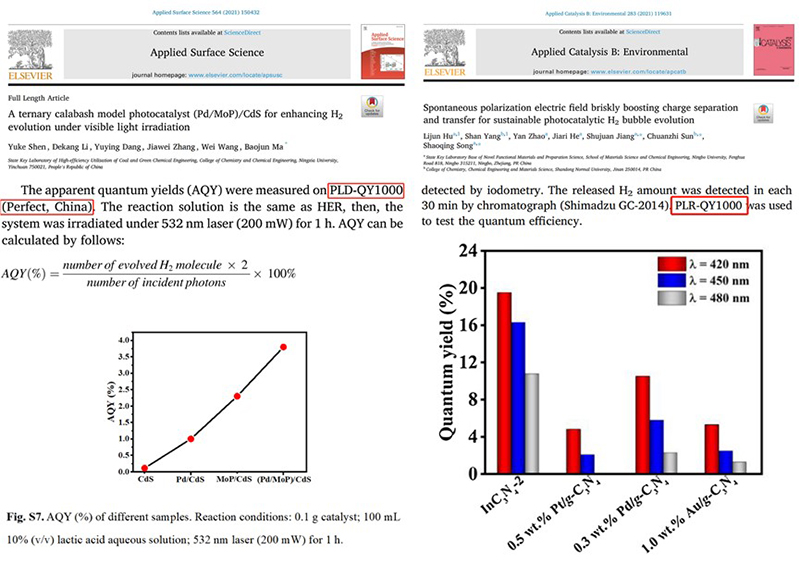
Figure 5. Classic cases of using the PLR-QY1000 photocatalytic quantum yield measurement system
The above content is a translation and summary based on reference materials. As my proficiency is limited, if there are any errors, kindly point them out!
[1]Qureshi Muhammad, Takanabe Kazuhiro *, Insights on measuring and reporting heterogeneous photocatalysis: efficiency definitions and setup examples[J]. Chemistry of Materials, 2017, 29, 158.
[2]Kisch Horst*, Bahnemann Detlef*, Best practice in photocatalysis: comparing rates or apparent quantum yields? [J]. The Journal of Physical Chemistry Letters, 2015, 6, 1907.
[3]Wang Zheng, Li Can, Domen Kazunari *, Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting[J]. Chemical. Society. Reviews, 2019, 48, 2109.
[4]Lin Huiwen, Chang Kun*, Ye Jinhua* et. al., Ultrafine nano 1T-MoS2 monolayers with NiOx as dual co-catalysts over TiO2 photoharvester for efficient photocatalytic hydrogen evolution[J]. Applied Catalysis B: Environmental, 2020, 279, 119387.
[5]Cheng Lei, Yue Xiaoyang, Xiang quanjun* et. al., Dual-single-atom tailoring with bifunctional integration for high-performance CO2 photoreduction[J]. Advanced. Materials. 2021, 33, 2105135.
[6]Zhao zhanfeng, Yang Dong, Jiang zhongyi* et. al., Nitrogenase-inspired mixed-valence MIL-53(FeII/FeIII) for photocatalytic nitrogen fixation[J]. Chemical Engineering Journal, 2020, 400, 125929.
[7]Shen Yuke, Li Dekang, Ma Baojun* et. al., A ternary calabash model photocatalyst (Pd/MoP)/CdS for enhancing H2 evolution under visible light irradiation[J]. Applied Surface Science, 2021, 564, 150432.
[8]Hu Lijun, Jiang Shujuan*,Song Shaoqing* et. al., Spontaneous polarization electric field briskly boosting charge separation and transfer for sustainable photocatalytic H2 bubble evolution[J]. Applied Catalysis B: Environmental, 2021, 283, 119631.