With the rapid growth of population and industrialization, global energy supply has increased dramatically. It is estimated that as of 2021, the total global energy consumption is approximately 600 EJ (1018 J), with over 80% of the energy supply coming from fossil fuels[1]. The use of fossil fuels leads to significant CO₂ emissions. The latest statistics show that the atmospheric CO₂ concentration has risen from 280 ppm before the industrial revolution to 416 ppm in 2020 (Figure 1)[2]. Excessive CO₂ emissions result in a range of issues, including global warming, glacier melting, and loss of biodiversity[3, 4]. Therefore, the utilization and conversion of CO₂ are becoming increasingly urgent.
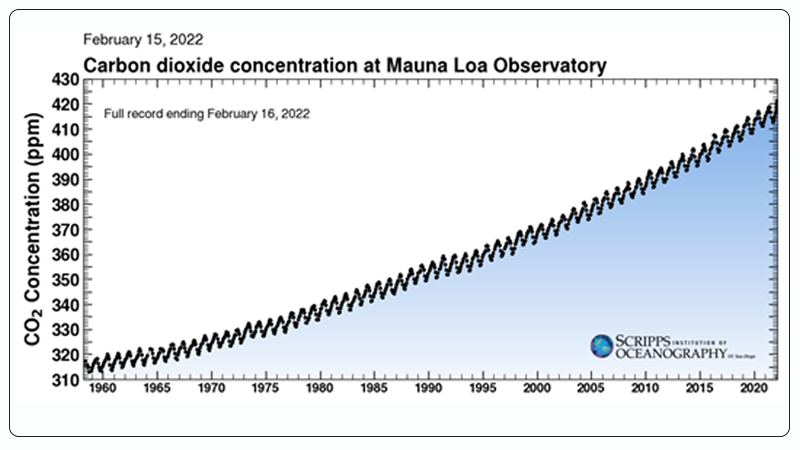
Figure 1. Global CO₂ Emissions from 1958 to Present[2].
Various technologies have been developed to convert CO₂ into hydrocarbons or high-value chemicals, including thermal catalysis[5,6], biocatalysis[7], photoelectrocatalysis[8, 9], electrocatalysis[10, 11], and photocatalytic reduction[12-14]. Among these methods, photocatalytic CO₂ reduction mimics natural photosynthesis, using solar energy and photocatalysts to catalytically convert CO₂ and H₂O (also known as artificial photosynthesis), enabling the efficient production of solar fuels and high-value chemicals such as methanol, ethanol, and hydrocarbons[15, 16], as shown in Figure 2. Therefore, photocatalytic CO₂ reduction is considered one of the most promising solutions to global energy and environmental challenges. In recent years, research related to photocatalytic CO₂ reduction has been increasing. Compared to traditional thermal catalysis, photocatalytic CO₂ reduction has the following four advantages[17]:
① Photocatalytic CO₂ reduction only relies on solar energy as an external energy source, which is renewable;[17]
② The reactants in photocatalytic CO₂ reduction are H₂O and CO₂, which are readily available;[17]
③ Photocatalytic CO₂ reduction operates under mild conditions, typically at room temperature and atmospheric pressure;[17]
④ Photocatalytic CO₂ reduction does not produce secondary pollution.[17]
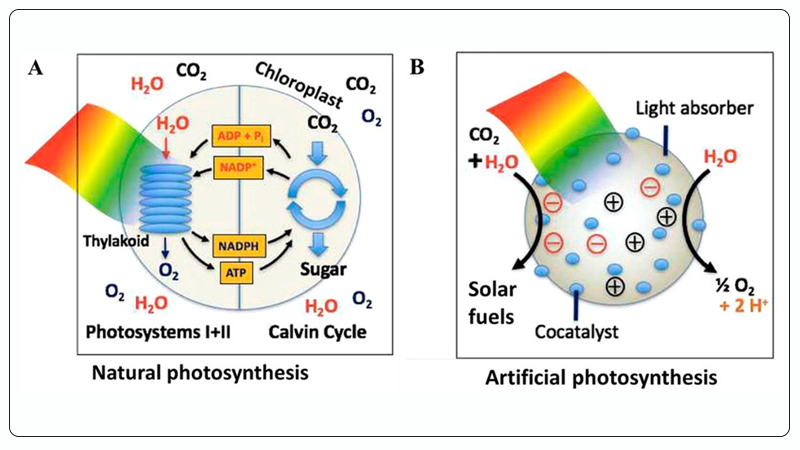
Figure 2. A - Natural Photosynthesis, B - Artificial Photosynthesis (Photocatalytic CO₂ Reduction)[16].
Photocatalytic CO₂ reduction is a complex, multi-step process. In general, the photocatalytic CO₂ reduction process involves the following three main steps[17]:
① Semiconductor photocatalysts are excited by light with energy greater than their bandgap width (Eg);
② Photogenerated electrons and holes are separated;
③ Photogenerated electrons migrate to the surface of the photocatalyst and react with CO₂ and H+ to form reduced products, while photogenerated holes react with H₂O to produce O₂. The entire photocatalytic CO₂ reduction process can occur in the gas phase or in a solution system[15].
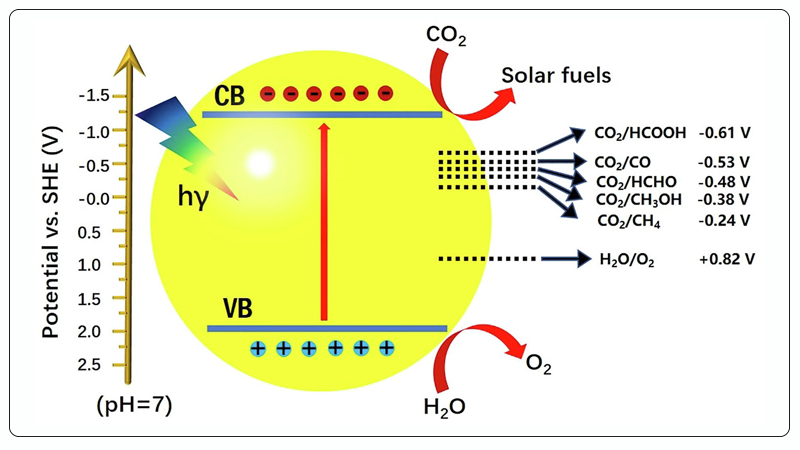
Figure 3. Schematic of Photocatalytic CO₂ Reduction[17].
Currently, the products of photocatalytic CO₂ reduction mainly include C1 compounds (CO, CH₄, CH₃OH, HCOOH) and C₂ compounds (C₂H₄, C₂H₆, C₃H₆, C₂H₅OH), among others.
In the field of chemical engineering, the products of photocatalytic CO₂ reduction have different applications[1, 18]:
① CO can be used as a feedstock gas for the Fischer-Tropsch synthesis to produce high-carbon chemicals;
② CH₄ is a major component of natural gas and can also be used in the reforming of CO₂;
③ Liquid products CH₃OH and HCOOH can be used in fuel cells, and CH₃OH can also be used as an additive in gasoline;
④ Ethylene is mainly used in the production of polyethylene and ethylene glycol, while ethane is used to prepare ethylene. Ethanol finds applications as a chemical solvent, in medicine, and as a fuel;
⑤ Ethylene glycol is used in the production of polyethylene terephthalate (the raw material for polyester).
The C=O bond energy in CO₂ can be as high as 750 kJ·mol-1, and its linear, symmetrical molecular structure makes it difficult to activate[15, 19]. Therefore, thermodynamically, CO₂ activation requires high-energy input. Due to efficiency and selectivity concerns, current research on photocatalytic CO₂ reduction is still in the laboratory stage.
At present, photocatalytic CO₂ reactions face several challenges[3, 15]:
① Limited light absorption capacity of the catalysts used in photocatalytic CO₂ reduction;
② Severe recombination of photogenerated charge carriers in photocatalytic CO₂ reduction;
③ Difficulty in adsorbing and activating CO₂;
④ Effective suppression of competing photocatalytic hydrogen evolution reaction against photocatalytic CO₂ reduction;
⑤ Need for improved stability of photocatalysts;
⑥ Development of a simplified synthesis process for photocatalytic CO₂ reduction catalysts;
⑦ Lack of extensive research on the mechanism of photocatalytic CO₂ reduction, making it difficult to control the selectivity of reduced products.
To address these issues, one approach is to enhance the efficiency of photocatalytic CO₂ reduction and improve the selectivity of target products through the design and synthesis of efficient catalysts. On the other hand, Perfectlight Technology hopes to collaborate with experts and researchers to develop well-designed reactors, optimize the photocatalytic CO₂ reduction process, and actively promote research related to photocatalytic CO₂ reduction.
[1] Lin Huiwen, Zhang Huabin*, Ye Jinhua* et. al., Toward solar-driven carbon recycling[J]. Joule, 2022, 6, 1-21.
[2] R. Keeling, Scripps institution of oceanography. scrippsco2.ucsd. edu/, 2022.
[3] Gong Eunhee, Ali Shahzad, In Su-Il* et. al., Solar fuels: research and development strategies to accelerate photocatalytic CO₂ conversion into hydrocarbon fuels[J]. Energy Environmental Science, 2022. DOI: 10.1039/d1ee0271
[4] Wang Wan-Hui*, Himeda Yuichiro*, Fujita Etsuko* et. al., CO₂ hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO₂ reduction[J]. Chemical Reviews, 2015, 115, 12936−12973.
[5] Rui Ning, Rodriguez José A.*, Liu Chang-Jun* et. al., Hydrogenation of CO₂ to methanol on a Auδ+-In₂O3–x catalyst[J]. ACS Catalysis, 2020, 10, 11307-11317.
[6] Hu Jingting, Wang Ye*, Deng Dehui* et. al., Sulfur vacancy-rich MoS₂ as a catalyst for the hydrogenation of CO₂ to methanol[J]. Nature Catalysis, 2021, 4, 242-250.
[7] Gong Fuyu, Zhang Yanping* et. al., Li Yin*, Biological carbon fixation: From natural to synthetic[J]. Journal of CO₂ Utilization, 2018, 28, 221-227.
[8] Dong Wan Jae, Lee Jong-Lam*, Zetian Mi*, et. al., Silver halide catalysts on GaN nanowires/Si heterojunction photocathodes for CO₂ reduction to syngas at high current density[J]. ACS Catalysis, 2022, 12, 2671-2680.
[9] Qin Yin, Hu Liuyong*, Gu Wenling*, et. al., Iron single-atom catalysts boost photoelectrochemical detection by integrating interfacial oxygen reduction and enzyme-mimicking activity[J]. ACS Nano, 2022. DOI: 10.1021/acsnano.1c10303.
[10] Liu Shuai, Chen Yu*, Liu Xijun*, et. al., Coordination environment engineering to boost electrocatalytic CO₂ reduction performance by introducing boron into single-Fe-atomic catalyst[J]. Chemial Engineering Journal, 2022. DOI: 10.1016/j.cej.2022.135294.
[11] Luc Wesley, Chen Jingguang G.*, Jiao Feng* et. al., SO₂-induced selectivity change in CO₂ electroreduction[J]. Journal of the American Chemical Society, 2019, 141, 9902-9909.
[12] Liu Qiong, Xiang Zhangmin*, Wang Fuxian*, et. al., Regulating the *OCCHO intermediate pathway towards highly selective photocatalytic CO₂ reduction to CH₃CHO over locally crystallized carbon nitride[J]. Energy Environmental Science, 2022, 15, 225.
[13] Li Fang, Yue Xiaoyang, Xiang Quanjun*, et. al., Targeted regulation of exciton dissociation in graphitic carbon nitride by vacancy modification for efficient photocatalytic CO₂ reduction[J]. Applied Catalysis B: Environmental, 2021, 292, 120179.
[14] Hao Jingxuan, Min Yulin*, Li Hexing*, et. al., Utilizing new metal phase nanocomposites deep photocatalytic conversion of CO₂ to C₂H₄[J]. Chemical Engineering Journal, 2021, 423, 130190.
[15] Shen Huidong, Peppel Tim*, Sun Zhenyu*, et. al., Photocatalytic reduction of CO₂ by metal-free-Based materials: recent advances and future perspective[J]. Solar RRL 2020, 4, 1900546.
[16] Li Xin, Yu Jiaguo*, Jaroniec Mietek* et. al., Cocatalysts for selective photoreduction of CO₂ into solar fuels[J]. Chemical Reviews, 2019, 119, 3962-4179.
[17] Fu Junwei, Yu Jiaguo*, Liu Min*, et. al., Product selectivity of photocatalytic CO₂ reduction reactions[J]. Materials Today, 2020, 32, 222-243.
[18] Albero Josep, Peng Yong, García Hermenegildo*, et. al., Photocatalytic CO₂ reduction to C2+ products[J]. ACS Catalysis, 2020, 10, 5734−5749.
[19] Liu Lizhen, Huang Hongwei*, Ma Tianyi*, et. al., Surface sites engineering on semiconductors to boost photocatalytic CO₂ reduction[J]. Nano Energy, 2020, 75, 104959.