Original content is not easy, if you wish to reproduce this article, please contact our staff and indicate the source in the reproduced article, otherwise infringement will be addressed!
With the rapid growth of population and industrialization, global energy demand has sharply increased. It is estimated that by 2021, the total global energy consumption is about 600 EJ (1018 J), with over 80% of energy supply coming from fossil fuels[1]. However, the use of fossil fuels leads to significant CO₂ emissions.
Recent statistical data shows that the atmospheric CO₂ concentration has risen from 280 ppm before the industrial revolution to 416 ppm in 2020 (Figure 1)[2]. Excessive CO₂ emissions result in a range of issues, including global warming, glacier melting, and loss of biodiversity[3, 4]. Therefore, the conversion and utilization of CO₂ have become urgent.
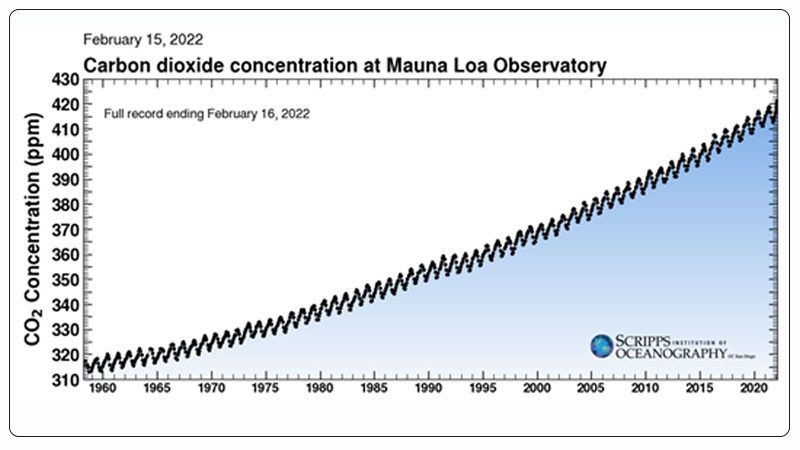
Figure 1. Global CO₂ Emissions from 1958 to Present[2].
Various technologies have been developed to convert CO₂ into hydrocarbons or high-value chemicals, including thermal catalysis[5,6], biocatalysis[7], photoelectrocatalysis[8, 9], electrocatalysis[10, 11], and photocatalytic reduction[12-14].
Among these methods, photocatalytic CO₂ reduction mimics natural photosynthesis, utilizing solar energy and photocatalysts to catalytically convert CO₂ and H₂O (also known as artificial photosynthesis), enabling the production of solar fuels and high-value chemicals such as methanol, ethanol, and hydrocarbons[15, 16], as shown in Figure 2. Therefore, photocatalytic CO₂ reduction is considered one of the most promising solutions for addressing global energy and environmental issues.
In recent years, research related to photocatalytic CO₂ reduction has been increasing. Compared to traditional thermal catalysis, photocatalytic CO₂ reduction offers four major advantages[17]:
① The external energy supply for photocatalytic CO₂ reduction is only solar energy, which is abundant and renewable;
② The reactants for photocatalytic CO₂ reduction are H₂O and CO₂, which are readily available;
③ The reaction conditions for photocatalytic CO₂ reduction are mild, typically at room temperature and atmospheric pressure;
④ Photocatalytic CO₂ reduction does not result in secondary pollution.
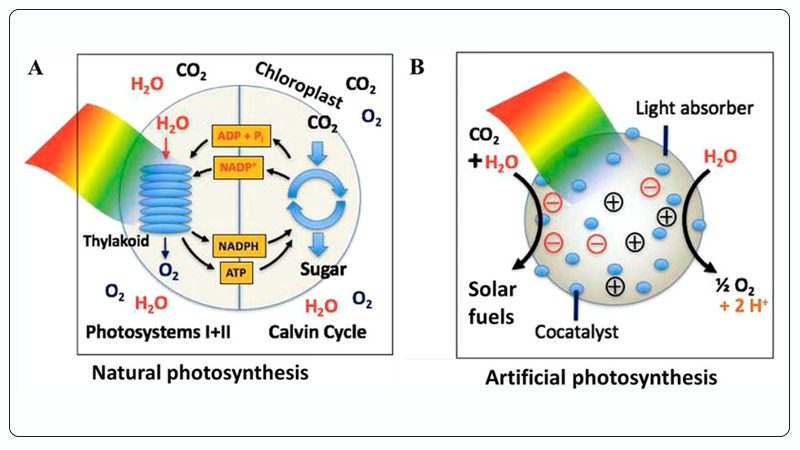
Figure 2. A Natural Photosynthesis, B Artificial Photosynthesis (Photocatalytic CO₂ Reduction Reaction)[16].
Photocatalytic CO₂ reduction is a complex multi-step process. In general, the photocatalytic CO₂ reduction process mainly involves the following three steps[18]:
① The semiconductor photocatalyst is excited by light with energy greater than its bandgap width (Eg);
② Photogenerated electrons and photogenerated holes are separated;
③ Photogenerated electrons migrate to the surface of the photocatalyst and react with CO₂ and H⁺ to form reduction products, while photogenerated holes react with H₂O to produce O₂.
The entire photocatalytic CO₂ reduction process can occur in the gas phase or in a liquid system[16].
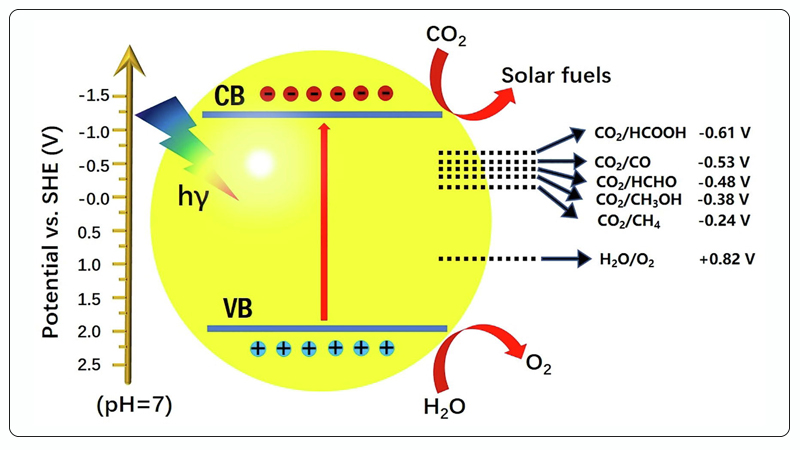
Figure 3. Photocatalytic CO₂ Reduction Diagram[17].
Currently, the products of photocatalytic CO₂ reduction mainly include C₁ products (CO, CH₄, CH₃OH, HCOOH) and C₂ products (C₂H₄, C₂H₆, C₃H₆, C₂H₅OH, etc.).
In the field of chemistry, the products of photocatalytic CO₂ reduction serve different purposes[1, 19]:
① CO can be used as feedstock for the Fischer-Tropsch synthesis to produce high-carbon chemicals;
② CH₄ is a major component of natural gas and can also be used in the reforming of CO₂;
③ Liquid products CH₃OH and HCOOH are mainly used in fuel cells, with CH₃OH also serving as a gasoline additive;
④ Ethylene is primarily used in the production of polyethylene and ethylene glycol, while ethane is used to prepare ethylene. Ethanol finds applications in chemical solvents, medicine, and fuel;
⑤ Ethylene glycol is used in the production of polyethylene terephthalate (the raw material for polyester).
The C=O bond in CO₂ has a high energy of up to 750 kJ·mol-1, and its linear symmetric molecular structure makes it challenging to activate[16, 20]. Therefore, thermodynamically, the activation of CO₂ requires high-energy input. Due to issues related to conversion efficiency and selectivity, current research on photocatalytic CO₂ reduction is still in the laboratory stage.
⑥ Developing a simplified synthesis process for photocatalytic CO2 reduction catalysts;
⑦ Lack of extensive research on the mechanism of photocatalytic CO2 reduction, making it difficult to control the selectivity of reduction products.
To address the above issues, on the one hand, it is possible to enhance the conversion efficiency of photocatalytic CO2 reduction reactions and improve the selectivity of target products by designing and synthesizing efficient catalysts. On the other hand, Perfectlight Technology hopes to engage in discussions and collaborate with experts and researchers to develop well-designed reactors and actively promote research related to photocatalytic CO2 reduction by optimizing the photocatalytic CO2 reduction process.
[1]Lin Huiwen, Zhang Huabin*, Ye Jinhua* et. al., Toward solar-driven carbon recycling[J]. Joule, 2022, 6, 1-21.
[2]R. Keeling, Scripps institution of oceanography. scrippsco2.ucsd. edu/, 2022.
[3]Gong Eunhee, Ali Shahzad, In Su-Il* et. al., Solar fuels: research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels[J]. Energy Environmental Science, 2022. DOI: 10.1039/d1ee0271
[4]Wang Wan-Hui*, Himeda Yuichiro*, Fujita Etsuko* et. al., CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction[J]. Chemical Reviews, 2015, 115, 12936−12973.
[5]Rui Ning, Rodriguez José A.*, Liu Chang-Jun* et. al., Hydrogenation of CO2 to methanol on a Auδ+-In2O3–x catalyst[J]. ACS Catalysis, 2020, 10, 11307-11317.
[6]Hu Jingting, Wang Ye*, Deng Dehui* et. al., Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol[J]. Nature Catalysis, 2021, 4, 242-250.
[7]Gong Fuyu, Zhang Yanping* et. al., Li Yin*, Biological carbon fixation: From natural to synthetic[J]. Journal of CO2 Utilization, 2018, 28, 221-227.
[8]Dong Wan Jae, Lee Jong-Lam*, Zetian Mi*, et. al., Silver halide catalysts on GaN nanowires/Si heterojunction photocathodes for CO2 reduction to syngas at high current density[J]. ACS Catalysis, 2022, 12, 2671-2680.
[9]Qin Yin, Hu Liuyong*, Gu Wenling*, et. al., Iron single-atom catalysts boost photoelectrochemical detection by integrating interfacial oxygen reduction and enzyme-mimicking activity[J]. ACS Nano, 2022. DOI: 10.1021/acsnano.1c10303.
[10]Liu Shuai, Chen Yu*, Liu Xijun*, et. al., Coordination environment engineering to boost electrocatalytic CO2 reduction performance by introducing boron into single-Fe-atomic catalyst[J]. Chemial Engineering Journal, 2022. DOI: 10.1016/j.cej.2022.135294.
[11]Luc Wesley, Chen Jingguang G.*, Jiao Feng* et. al., SO2-induced selectivity change in CO2 electroreduction[J]. Journal of the American Chemical Society, 2019, 141, 9902-9909.
[12]Liu Qiong, Xiang Zhangmin*, Wang Fuxian*, et. al., Regulating the *OCCHO intermediate pathway towards highly selective photocatalytic CO2 reduction to CH3CHO over locally crystallized carbon nitride[J]. Energy Environmental Science, 2022, 15, 225.
[13]Li Fang, Yue Xiaoyang, Xiang Quanjun*, et. al., Targeted regulation of exciton dissociation in graphitic carbon nitride by vacancy modification for efficient photocatalytic CO2 reduction[J]. Applied Catalysis B: Environmental, 2021, 292, 120179.
[14]Hao Jingxuan, Min Yulin*, Li Hexing*, et. al., Utilizing new metal phase nanocomposites deep photocatalytic conversion of CO2 to C2H4[J]. Chemical Engineering Journal, 2021, 423, 130190.
[15]Shen Huidong, Peppel Tim*, Sun Zhenyu*, et. al., Photocatalytic reduction of CO2 by metal-free-Based materials: recent advances and future perspective[J]. Solar RRL 2020, 4, 1900546.
[16]Li Xin, Yu Jiaguo*, Jaroniec Mietek* et. al., Cocatalysts for selective photoreduction of CO2 into solar fuels[J]. Chemical Reviews, 2019, 119, 3962-4179.
[17]Fu Junwei, Yu Jiaguo*, Liu Min*, et. al., Product selectivity of photocatalytic CO2 reduction reactions[J]. Materials Today, 2020, 32, 222-243.
[18]Albero Josep, Peng Yong, García Hermenegildo*, et. al., Photocatalytic CO2 reduction to C2+ products[J]. ACS Catalysis, 2020, 10, 5734−5749.
[19]Liu Lizhen, Huang Hongwei*, Ma Tianyi*, et. al., Surface sites engineering on semiconductors to boost photocatalytic CO2 reduction[J]. Nano Energy, 2020, 75, 104959.
The above section has been translated and summarized by the author based on reference materials. The author's proficiency is limited, so any errors are welcome to be pointed out by everyone!